Chemists and physicists know that at thermodynamic equilibrium, the energy in a system of small particles (atoms, molecules, nanoparticles, …) is distributed according to the Boltzmann distribution. With solutions of nanometer-sized gold nanospheres in our laboratory fridge, we have been able to photograph such Boltzmann equilibrium distributions directly.
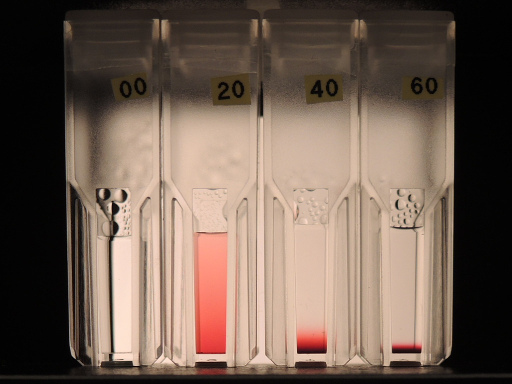
Gold nanoparticle solutions in water at sedimentation equilibrium (20, 40, 60 nm diameter).
This is one of the results in our latest publication in the Particle journal, which has its roots in recurring discussions with fellow chemists on whether specific solutions of nanoparticles should decant, or not, and if yes, then how long it would take (long!), and why in the other’s lab the particles do not settle (thermal convection)… etc.
We learned that it is worthwhile to think (again!) about the sedimentation of small particles, when one is working with functionalized nanoparticles. And look at the photos!
- Read more in the original publication.
- Prof. Steven Abbott has made a Javascript application that interactively shows the evolution of the concentration profile during nanoparticle sedimentation. It numerically calculates solutions to the Mason-Weaver equation using a Javascript implementation of the numerical scheme from our paper .